Factors in Genetic Alterations in DNA Repair Pathways and Their Effects on Endometrial Cancer
DOI:
https://doi.org/10.56226/144Keywords:
Endometrial Cancer, Genetic Mutations, DNA Repair Genes, Base Mismatch Repair, Microsatellite InstabilityAbstract
Introduction: Endometrial cancer is the most common gynecological neoplasm, with its origins closely linked to genetic alterations. This study highlights mutations in genes involved in DNA repair, especially the mismatch repair system and the BRCA1/2 genes, as central elements in the development of the disease, its molecular profile, and response to treatments. Understanding these genetic impacts is crucial to improving diagnostic methods and driving the development of personalized therapeutic approaches.
Methodology: A literature review was conducted, searching for articles in the PubMed and Scopus databases. Inclusion criteria included molecular, clinical, and epidemiological studies that established a correlation between mutations in DNA repair genes and endometrial cancer, published in the last ten years. The selected studies were analyzed to gather information on the prevalence of mutations in the mismatch repair system and BRCA1/2 genes, the frequency of microsatellite instability, the clinical and molecular profiles of the tumors, and the impact of these alterations on the response to therapies, especially immunotherapy.
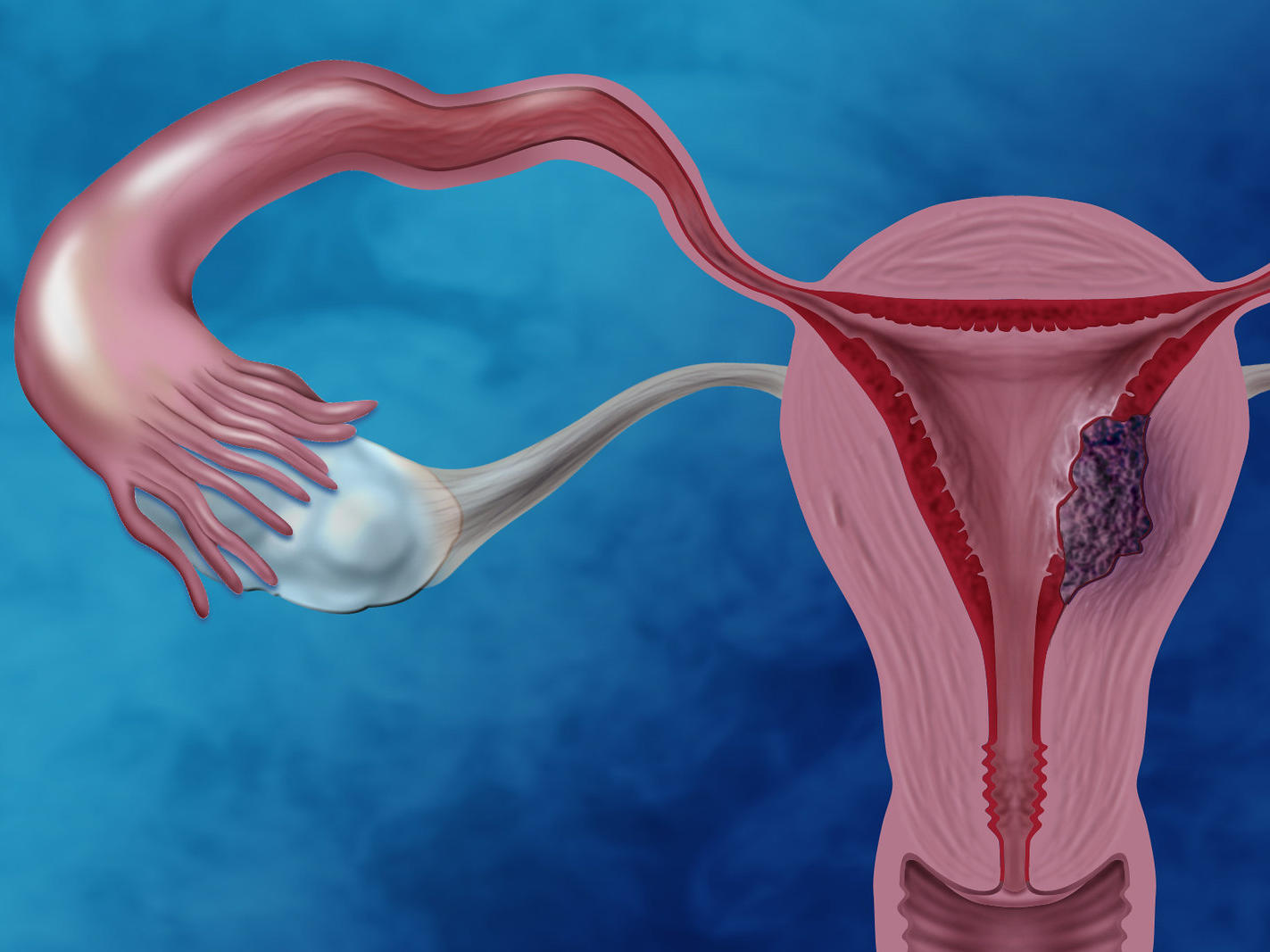
Results: The results of the review indicate that mismatch repair deficiency, caused by germline mutations or epigenetic methylation, represents a relevant molecular event in approximately 20 to 30% of endometrial cancer cases. This deficiency is associated with microsatellite instability and a hypermutated phenotype, characterized by a high mutational burden. This condition makes the tumor more immunogenic, favoring a positive response to immunotherapy with immune checkpoint inhibitors. Furthermore, germline mutations in the MMR genes are linked to Lynch Syndrome, which significantly increases the risk of developing endometrial cancer in young women. Mutations in the BRCA1/2 genes have also been identified, especially in the more aggressive serous subtype, suggesting a role in homologous recombination deficiency and tumor progression.
Conclusion: Genetic alterations in genes responsible for DNA repair play a key role in the molecular biology of endometrial cancer. They not only determine specific molecular subtypes of the disease but also serve as valuable indicators for diagnosis, prognostic assessment, and patient selection for targeted therapies.
References
Chow, R. D., Michaels, T., Bellone, S., Hartwich, T. M. P., Bonazzoli, E., Iwasaki, A., Song, E., & Santin, A. D. (2023). Distinct Mechanisms of Mismatch-Repair Deficiency Delineate Two Modes of Response to Anti-PD-1 Immunotherapy in Endometrial Carcinoma. Cancer discovery, 13(2), 312–331. https://doi.org/10.1158/2159-8290.CD-22-0686
Coleman RL, Garside J, Hurteau J, Nguyen J, Kobayashi M. Treatment Patterns and Outcomes Among Patients With Advanced or Recurrent Endometrial Cancer Initiating First-Line Therapy in the United States. J Health Econ Outcomes Res. 2023 Oct 27;10(2):82-90. doi: 10.36469/001c.87853. PMID: 37905183; PMCID: PMC10613433.
Ferlay, J., Colombet, M., Soerjomataram, I., Parkin, D. M., Piñeros, M., Znaor, A., & Bray, F. (2021). Cancer statistics for the year 2020: An overview. International journal of cancer, 10.1002/ijc.33588. Advance online publication. https://doi.org/10.1002/ijc.33588
Galant, N., Krawczyk, P., Monist, M., Obara, A., Gajek, Ł., Grenda, A., Nicoś, M., Kalinka, E., & Milanowski, J. (2024). Molecular Classification of Endometrial Cancer and Its Impact on Therapy Selection. International journal of molecular sciences, 25(11), 5893. https://doi.org/10.3390/ijms25115893
Kempers, M. J., Kuiper, R. P., Ockeloen, C. W., Chappuis, P. O., Hutter, P., Rahner, N., Schackert, H. K., Steinke, V., Holinski-Feder, E., Morak, M., Kloor, M., Büttner, R., Verwiel, E. T., van Krieken, J. H., Nagtegaal, I. D., Goossens, M., van der Post, R. S., Niessen, R. C., Sijmons, R. H., Kluijt, I., … Ligtenberg, M. J. (2011). Risk of colorectal and endometrial cancers in EPCAM deletion-positive Lynch syndrome: a cohort study. The Lancet. Oncology, 12(1), 49–55. https://doi.org/10.1016/S1470-2045(10)70265-5
Kennedy, L. B., & Salama, A. K. S. (2020). A review of cancer immunotherapy toxicity. CA: A Cancer Journal for Clinicians, 70(2), 86–104. https://doi.org/10.3322/caac.21596
Marabelle, A., Fakih, M., Lopez, J., Shah, M., Shapira-Frommer, R., Nakagawa, K., Chung, H. C., Kindler, H. L., Lopez-Martin, J. A., Miller, W. H., Jr, Italiano, A., Kao, S., Piha-Paul, S. A., Delord, J. P., McWilliams, R. R., Fabrizio, D. A., Aurora-Garg, D., Xu, L., Jin, F., Norwood, K., … Bang, Y. J. (2020). Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. The Lancet. Oncology, 21(10), 1353–1365. https://doi.org/10.1016/S1470-2045(20)30445-9
Mauro, B. C. N., & Carvalho, R. S. (2023). O potencial revolucionário da imunoterapia no tratamento do câncer de endométrio avançado/recorrente com deficiência de enzimas de reparo ou alta instabilidade de microssatélite. Brazilian Journal of Health Review, 6(6), 32930–32947. https://doi.org/10.34119/bjhrv6n6-490
Mendiola, M., Heredia-Soto, V., Ruz-Caracuel, I., Baillo, A., Ramon-Patino, J. L., Escudero, F. J., Miguel, M., Pelaez-Garcia, A., Hernandez, A., Feliu, J., Hardisson, D., & Redondo, A. (2023). Comparison of Methods for Testing Mismatch Repair Status in Endometrial Cancer. International journal of molecular sciences, 24(19), 14468. https://doi.org/10.3390/ijms241914468
Oaknin, A., Tinker, A. V., Gilbert, L., Samouëlian, V., Mathews, C., Brown, J., Barretina-Ginesta, M. P., Moreno, V., Gravina, A., Abdeddaim, C., Banerjee, S., Guo, W., Danaee, H., Im, E., & Sabatier, R. (2021). Clinical activity and safety of the anti-PD-1 monoclonal antibody dostarlimab for patients with recurrent or advanced dMMR endometrial cancer. Future oncology (London, England), 17(29), 3781–3785. https://doi.org/10.2217/fon-2021-0598
Oaknin, A., Gilbert, L., Tinker, A. V., Brown, J., Mathews, C., Press, J., Sabatier, R., O'Malley, D. M., Samouelian, V., Boni, V., Duska, L., Ghamande, S., Ghatage, P., Kristeleit, R., Leath, C. I. I. I., Guo, W., Im, E., Zildjian, S., Han, X., Duan, T., … Pothuri, B. (2022). Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: interim results from GARNET-a phase I, single-arm study. Journal for immunotherapy of cancer, 10(1), e003777. https://doi.org/10.1136/jitc-2021-003777
O'Malley, D. M., Bariani, G. M., Cassier, P. A., Marabelle, A., Hansen, A. R., De Jesus Acosta, A., Miller, W. H., Jr, Safra, T., Italiano, A., Mileshkin, L., Xu, L., Jin, F., Norwood, K., & Maio, M. (2022). Pembrolizumab in Patients With Microsatellite Instability-High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 40(7), 752–761. https://doi.org/10.1200/JCO.21.01874
Peltomäki, P., Nyström, M., Mecklin, J. P., & Seppälä, T. T. (2023). Lynch Syndrome Genetics and Clinical Implications. Gastroenterology, 164(5), 783–799. https://doi.org/10.1053/j.gastro.2022.08.058
Riedinger, C. J., Esnakula, A., Haight, P. J., Suarez, A. A., Chen, W., Gillespie, J., Villacres, A., Chassen, A., Cohn, D. E., Goodfellow, P. J., & Cosgrove, C. M. (2024). Characterization of mismatch-repair/microsatellite instability-discordant endometrial cancers. Cancer, 130(3), 385–399. https://doi.org/10.1002/cncr.35030
Siegel, R. L., Miller, K. D., Wagle, N. S., & Jemal, A. (2023). Cancer statistics, 2023. CA: a cancer journal for clinicians, 73(1), 17–48. https://doi.org/10.3322/caac.21763
Smith, M. L., & Fornace, A. J., Jr (1995). Genomic instability and the role of p53 mutations in cancer cells. Current opinion in oncology, 7(1), 69–75.
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians, 71(3), 209–249. https://doi.org/10.3322/caac.21660
Vermij, L., Léon-Castillo, A., Singh, N., Powell, M. E., Edmondson, R. J., Genestie, C., Khaw, P., Pyman, J., McLachlin, C. M., Ghatage, P., de Boer, S. M., Nijman, H. W., Smit, V. T. H. B. M., Crosbie, E. J., Leary, A., Creutzberg, C. L., Horeweg, N., Bosse, T., & TransPORTEC consortium (2022). p53 immunohistochemistry in endometrial cancer: clinical and molecular correlates in the PORTEC-3 trial. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc, 35(10), 1475–1483. https://doi.org/10.1038/s41379-022-01102-x
Wild, C. P., Weiderpass, E., & Stewart, B. W. (Eds.). (2020). World Cancer Report: Cancer research for cancer prevention. International Agency for Research on Cancer.
Yokoyama, T., Takehara, K., Sugimoto, N., Kaneko, K., Fujimoto, E., Okazawa-Sakai, M., Okame, S., Shiroyama, Y., Yokoyama, T., Teramoto, N., Ohsumi, S., Saito, S., Imai, K., & Sugano, K. (2018). Lynch syndrome-associated endometrial carcinoma with MLH1 germline mutation and MLH1 promoter hypermethylation: a case report and literature review. BMC cancer, 18(1), 576. https://doi.org/10.1186/s12885-018-4489-0
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2026 The Authors

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution (CC-BY) 4.0 License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this journal.



 Start-up >
Start-up >